But Stevens was confident Harrell could hold up her end of the deal.
“She told me, ‘You cannot do drugs and do your pain meds,’” Harrell recounted on a Monday evening in October. So, “I’m no longer on cocaine.”
Stevens’ approach to patient care has won her awards and nominations in medicine, community service, and humanism. Instead of seeing patients in binaries — addicted or sober, with a positive or negative drug test — she measures progress on a spectrum. Are they showering daily, cooking with their families, using less fentanyl than the day before?
But not everyone agrees with this flexible approach that prioritizes working with patients on their goals, even if abstinence isn’t one of them. And it came to a head in the summer of 2024.
“The same things I was high-fived for thousands of times — suddenly that was bad,” Stevens said.
Flexible Care or Zero Tolerance?
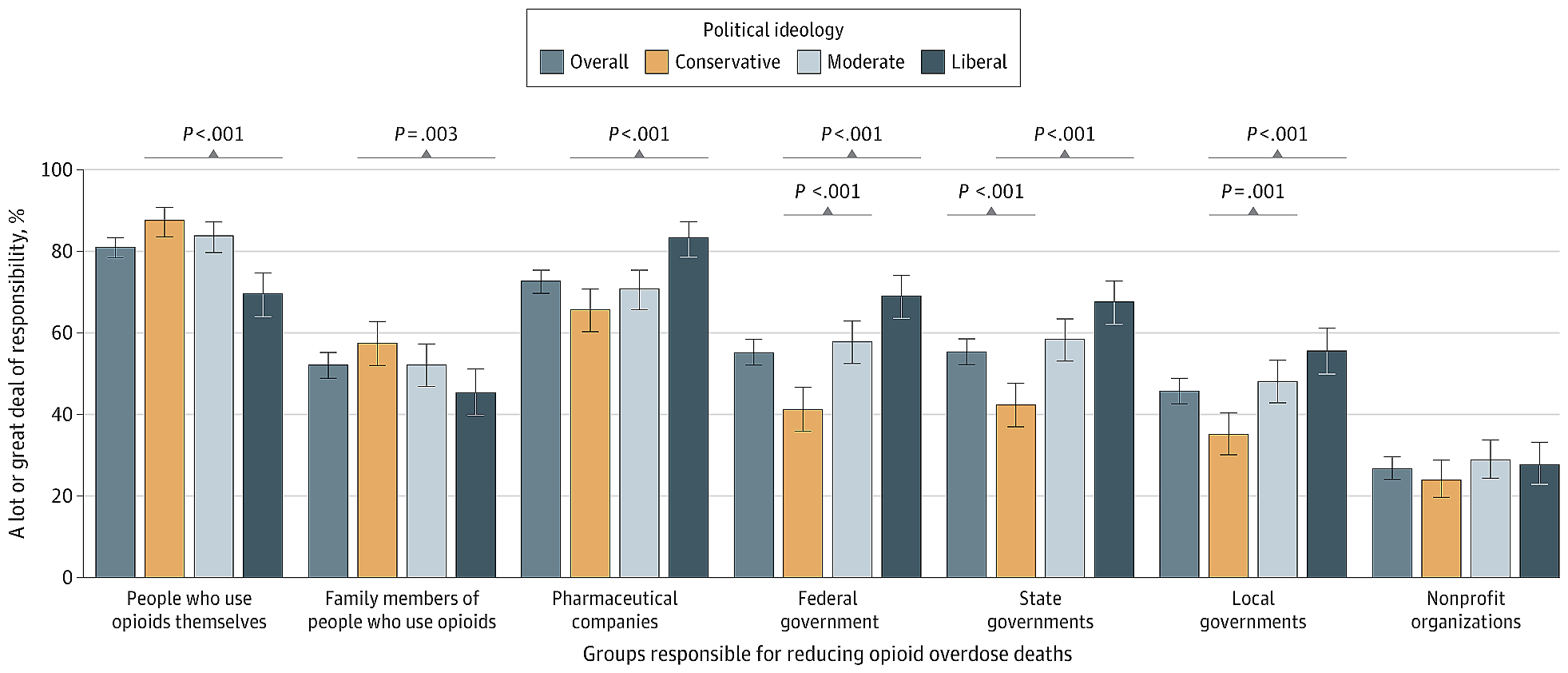
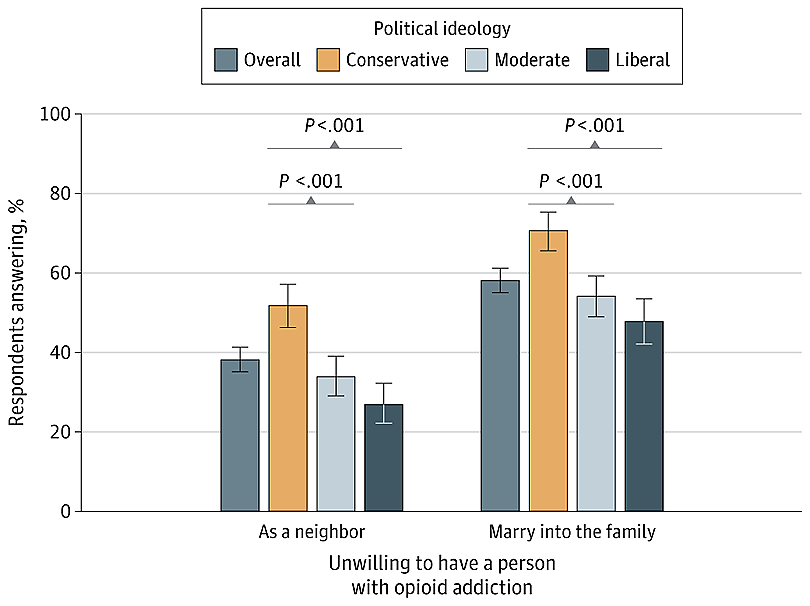
More than 80% of Americans who need substance use treatment don’t receive it, national data shows. Barriers abound: high costs, lack of transportation, clinic hours that are incompatible with jobs, fear of being mistreated.
Some doctors had been trying to ease the process for years. Covid-19 accelerated that trend. Telehealth appointments, fewer urine drug tests, and medication refills that last longer became the norm.
The result?
“Patients did OK and we actually reached more people,” said Brian Hurley, immediate past president of the American Society of Addiction Medicine. The organization supports continuing flexible practices, such as helping patients avoid withdrawal symptoms by prescribing higher-than-traditional doses of addiction medication and focusing on recovery goals other than abstinence.
But some doctors prefer traditional approaches that range from zero tolerance for patients using illegal drugs to setting stiff consequences for those who don’t meet their doctors’ expectations. For example, a patient who tests positive for street drugs while getting outpatient care would be discharged and told to go to residential rehab.
Proponents of this method fear loosening restrictions could be a slippery slope that ultimately harms patients. They say continuing to prescribe painkillers, for example, to people using illicit substances long-term could normalize drug use and hamper the goal of getting people off illegal drugs.
Progress should be more than keeping patients in care, said Keith Humphreys, a Stanford psychologist, who has treated and researched addiction for decades and supports involuntary treatment.
“If you give addicted people lots of drugs, they like it, and they may come back,” he said. “But that doesn’t mean that that is promoting their health over time.”
Flexible practices also tend to align with harm reduction, a divisive approach that proponents say keeps people who use drugs safe and that critics — including the Trump administration — say enables illegal drug use.
The debate is not just philosophical. For Stevens and her patients, it came to bear on the streets of New Orleans.
‘Unconventional’ Prescribing
In the summer of 2024, supervisors started questioning Stevens’ approach.
In emails reviewed by KFF Health News, they expressed concerns about her prescribing too many pain pills, a mix of opioids and other controlled substances to the same patients, and high doses of buprenorphine, a medication considered the gold standard to treat opioid addiction.
Supervisors worried Stevens wasn’t doing enough urine drug tests and kept treating patients who used illicit drugs instead of referring them to higher levels of care.
“Her prescribing pattern appears unconventional compared to the local standard of care,” the hospital’s chief medical officer at the time wrote to Stevens’ supervisor, Benjamin Springgate. “Note that this is the only standard of care which would likely be considered should a legal concern arise.”
Springgate forwarded that email to Stevens and encouraged her to refer more patients to methadone clinics, intensive outpatient care, and inpatient rehab.
Stevens understood the general practice but couldn’t reconcile it with the reality her patients faced. How would someone living in a tent, fearful of losing their possessions, trek to a methadone clinic daily?
Stevens sent her supervisors dozens of research studies and national treatment guidelines backing her flexible approach. She explained that if she stopped prescribing the medications of concern, patients might leave the health system, but they wouldn’t disappear.
“They just wouldn’t be getting care and perhaps they’d be dead,” she said in an interview with KFF Health News.
Both University Medical Center and LSU Health New Orleans, which employs physicians at the hospital, declined repeated requests for interviews. They did not respond to detailed questions about addiction treatment or Stevens’ practices.
Instead, they provided a joint statement from Richard DiCarlo, dean of the LSU Health New Orleans School of Medicine, and Jeffrey Elder, chief medical officer of University Medical Center New Orleans.
“We are not at liberty to comment publicly on internal personnel issues,” they wrote.
“We recognize that addiction is a serious public health problem, and that addiction treatment is a challenge for the healthcare industry,” they said. “We remain dedicated to expanding access to treatment, while upholding the highest standard of care and safety for all patients.”
Not Black-and-White
KFF Health News shared the complaints against Stevens and the responses she’d written for supervisors with two addiction medicine doctors outside of Louisiana, who had no affiliation with Stevens. Both found her practices to be within the bounds of normal addiction care, especially for complex patients.
Stephen Loyd, an addiction medicine doctor and the president of Tennessee’s medical licensing board, said doctors running pill mills typically have sparse patient notes that list a chief complaint of pain. But Stevens’ notes detailed patients’ life circumstances and the intricate decisions she was making with them.
“To me, that’s the big difference,” Loyd said.
Some people think the “only good answer is no opioids,” such as oxycodone or hydrocodone, for any patients, said Cara Poland, an addiction medicine doctor and associate professor at Michigan State University. But patients may need them — sometimes for things like cancer pain — or require months to lower their doses safely, she said. “It’s not as black-and-white as people outside our field want it to be.”
Humphreys, the Stanford psychologist, had a different take. He did not review Stevens’ case but said, as a general practice, there are risks to prescribing painkillers long-term, especially for patients using today’s lethal street drugs too.
Overprescribing fueled the opioid crisis, he said. “It’s not going to go away if we do that again.”
‘The Thing That Kills People’
After months of tension, Stevens’ supervisors told her on March 10 to stop coming to work. The hospital was conducting a review of her practices, they said in an email viewed by KFF Health News.
Overnight, hundreds of her patients were moved to other providers.
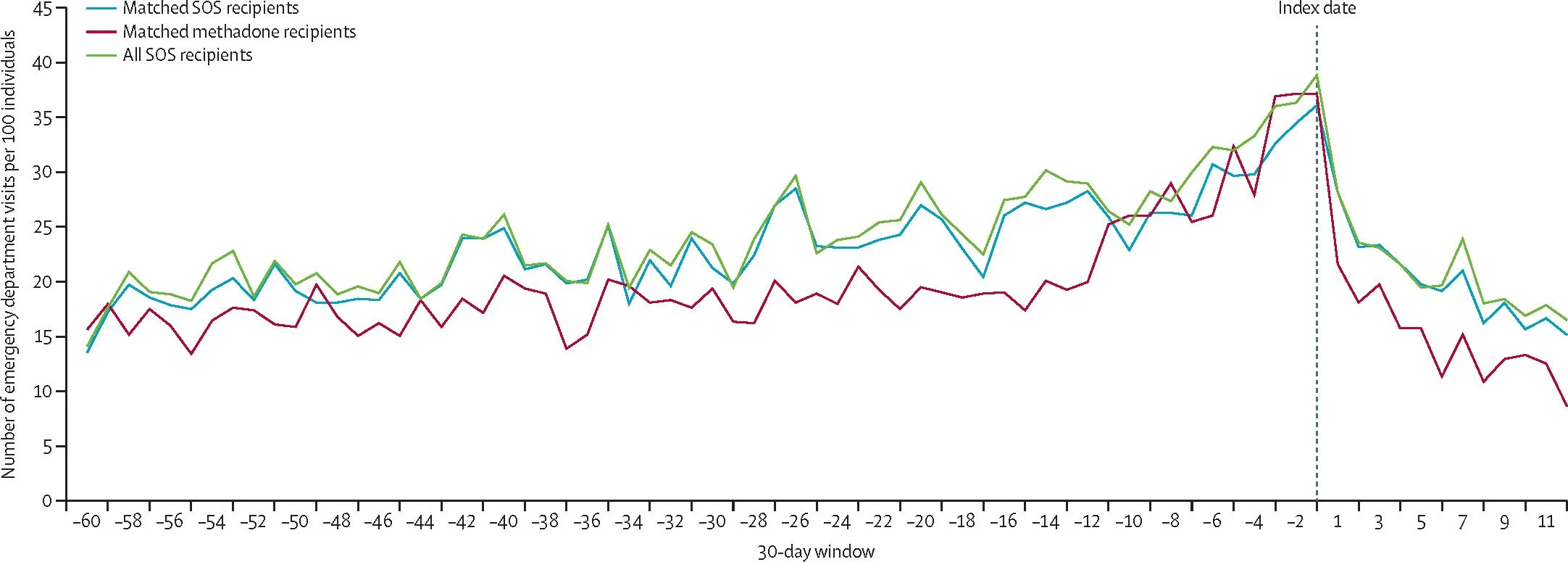
Luka Bair had been seeing Stevens for three years and was stable on daily buprenorphine.
After Stevens’ departure, Bair was left without medication for three days. The withdrawal symptoms were severe — headache, nausea, muscle cramps.
“I was just in physical hell,” said Bair, who works for the National Harm Reduction Coalition and uses they/them pronouns.
Although Bair eventually got a refill, Springgate, Stevens’ supervisor, didn’t want to continue the regimen long-term. Instead, Springgate referred Bair to more intensive and residential programs, citing Bair’s intermittent use of other drugs, including benzodiazepines and cocaine, as markers of high risk. Bair “requires a higher level of care than our clinic reasonably can offer,” Springgate wrote in patient portal notes reviewed by KFF Health News.
But Bair said daily attendance at those programs was incompatible with their full-time job. They left the clinic, with 30 days to find a new doctor or run out of medication again.
“This is the thing that kills people,” said Bair, who eventually found another doctor willing to prescribe.
Springgate did not respond to repeated calls and emails requesting comment.
University Medical Center and LSU Health New Orleans did not answer questions about discharging Stevens’ patients.
‘Patients Will Die Without Her’
About a month after Stevens was told to stay home, Haley Beavers Khoury, a medical student who worked with her, had collected nearly 100 letters from other students, doctors, patients, and homelessness service providers calling for Stevens’ return.
One student wrote, “Make no mistake — some of her patients will die without her.” A nun from the Daughters of Charity, which ran the hospital’s previous incarnation, called Stevens a “lifeline” for vulnerable patients.
Beavers Khoury said she sent the letters to about 10 people in hospital and medical school leadership. Most did not respond.
In May, the hospital’s review committee determined Stevens’ practices fell “outside of the acceptable community standards” and constituted “reckless behavior,” according to a letter sent to Stevens.
The hospital did not answer KFF Health News’ questions about how it reached this conclusion or if it identified any patient harm.
Meanwhile, Stevens had secured a job at another New Orleans hospital. But because her resignation came amid the ongoing investigation, University Medical Center said it was required to inform the state’s medical licensing board.
The medical board began its own investigation — a development that eventually cost Stevens the other job offer.
In presenting her side to the medical board, Stevens repeated many arguments she’d made before. Yes, she was prescribing powerful medications. No, she wasn’t making clinical decisions based on urine drug tests. But national addiction organizations supported such practices and promoted tailoring care to patients’ circumstances, she said. Her response included a 10-page bibliography with 98 citations.
Abandoning People
The board’s investigation into Stevens is ongoing. Its website shows no action taken against her license as of late December.
The board declined to comment on both Stevens’ case and its definition of appropriate addiction treatment.
In October, Stevens moved to the Virgin Islands to work in internal medicine at a local hospital. She said she’s grateful for the welcoming locals and the financial stability to support herself and her parents.
But it hurts to think of her former patients in New Orleans.
Before leaving, Stevens packed away handwritten letters from several of them — one was 15 pages long, written in alternating green and purple marker — in which they shared childhood traumas and small successes they had while in treatment with her.
Stevens doesn’t know what happened to those patients after she left.
She believes the scrutiny of her practice centers on liability more than patient safety.
But, she said, “liability is in abandoning people too.”
KFF Health News is a national newsroom that produces in-depth journalism about health issues