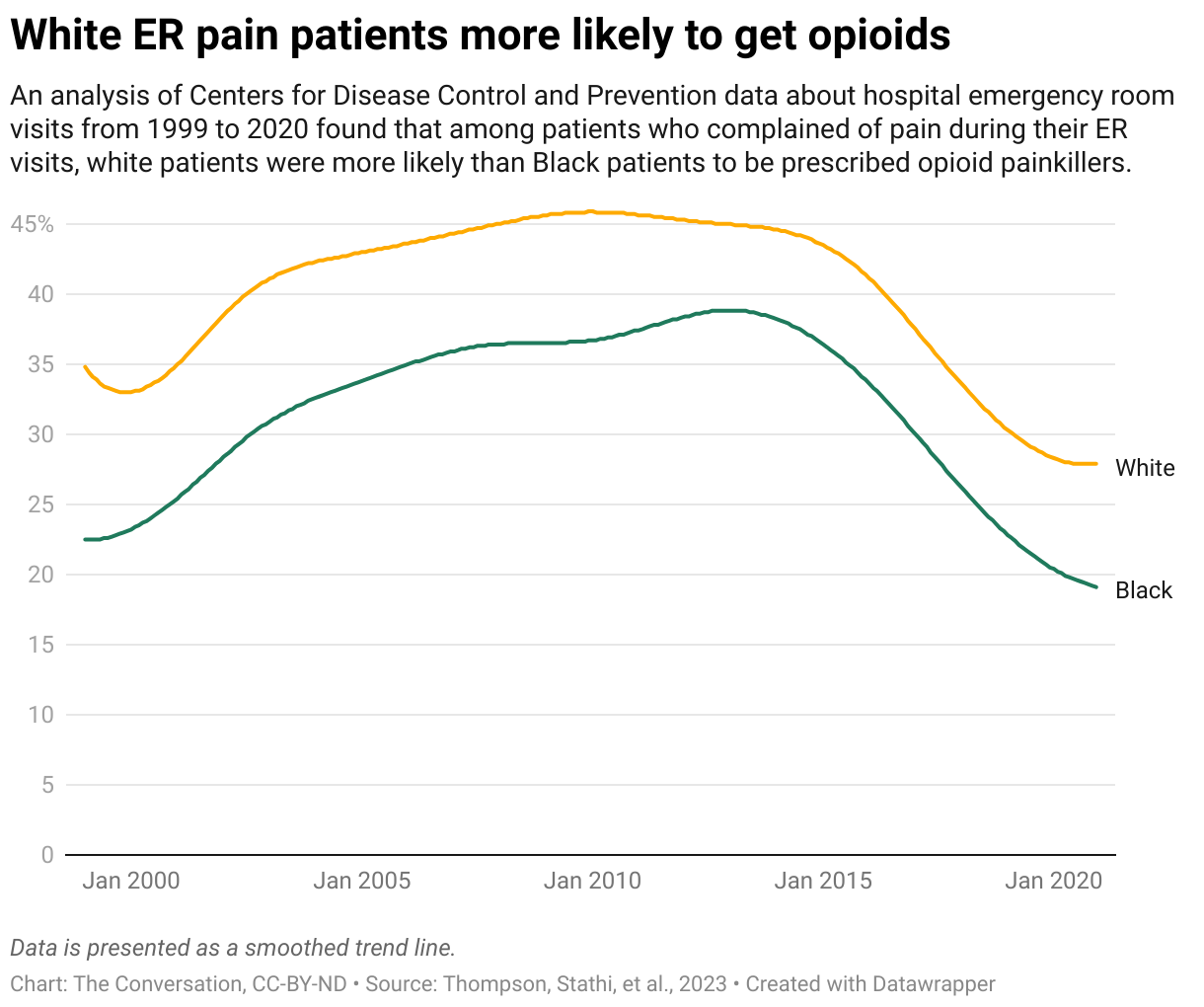
Doctor Bison’s Fables: The Crowded Exam Room
/By Pat Anson, PNN Editor
Dr. Mark Ibsen is a Montana physician whose license was suspended in 2016 by the state medical board for “overprescribing” opioids and poor record keeping. A state judge later reversed the suspension, ruling the medical board made numerous errors.
Ibsen has since become a strong proponent of medical marijuana and a leading advocate in the pain community. He’s published a new book, “Dr. Bison’s Fables,” using animals as lead characters in a series of stories to convey how many pain patients in the U.S. lack proper treatment and some are in crisis.
This interview with Ibsen has been edited for content and clarity.
PNN: Who is “Doctor Bison’s Fables” intended for and what is your goal? What is the message you're trying to get across?
Dr. Mark Ibsen
IBSEN: I'm not really in the book selling business. I'm in the restoration of the sacred physician-patient relationship business. But all of my screaming from the rooftops about it for the last 12 years has made little progress.
I was talking to the publisher and he was saying if you write a book about what's going on in medicine today, the pain crisis and catastrophe of it, you'll have a readership of 50,000. If you write a book that talks about the dynamics of what's going on, and put it in the form of a parable or a fable that has a bigger meaning to it, your audience will expand.
When this approach was suggested to me, I rejected it completely out of hand. I said I just want to tell what's going on because the house is on fire. But I slowly came to look at this idea as maybe he's onto something. Maybe Aesop's Fables had some good things to say, maybe the Brothers Grimm had some good things to say. So I thought, okay, let's add some folksiness to this. Give it a Burl Ives approach.
PNN: When you tell your fables in the book, animals are the key characters. Why did you choose them?
IBSEN: The animals are the protagonists. I chose Dr. Bison because of the bullheadedness of the character. As you can see on the book cover, he's examining this cute little otter on the exam table. And the weasel guy with the clipboard is scowling at him and evaluating how he's doing his job and if he's doing it effectively or quickly enough.
The weasel is like an interloper in the exam room, which has been my persistent complaint. We have too many guests in the exam room that have a vote into what's going on between the doctor and the patient. We have an insurance bureaucrat deciding what you can or can't prescribe, and a pharmacist deciding if the prescription is legitimate.
Then there's a law enforcement officer who's looking at whether a crime is being committed. Then there's a DEA agent who may or may not have a college education, who's looking at patterns and matrices. Is Dr. Bison prescribing more than other doctors?
Then there's legislators, like the legislators in the state of Ohio that legislated exactly how much I could get for the pain in my chest after it was split open during heart bypass surgery at the Cleveland Clinic.
So it's really crowded in the exam room now.
PNN: You want to get across the point that you can't treat all patients the same way. Everyone is different. How do you communicate that in your fable scenario?
IBSEN: I think it's basically species dependent. By that I mean that different species have different personal attributes. A mouse is going to attack a problem much different than a bison would. Or an herbivore will attack a problem more than a carnivore will.
I guess you could call it a menagerie. There's a bison and a cougar. There's a possum. There's a wise llama. There's a viper in the chapter about breeding resentment. There's a coyote, the trickster who has a lawyer's briefcase and there's a crow. And then the elk. Elk handle their conflicts by keeping the herd intact, surrounding the weakest member so the wolves can't get to them.
My goal is to is to make this seemingly light and then develop it. Story development pulls them in and readers finish the story. And then they're left with curiosity about the pain crisis, rather than revulsion or rejection or resistance or argumentation.
PNN: Take us to the epilogue and the message that you really want to get across to the reader.
IBSEN: I guess I would say that I didn't leave medicine, medicine left me. I don't want to project some righteous indignation about it. I'm sad about it more than anything else. I don't think this is limited just to the patient-physician relationship. I think it's a cultural phenomenon. And it's just manifested in medicine in this way.
We now have patients that cannot tell their doctor the truth. It's sort of like a teenager who has to lie to their parents. It's like a patriarchal system. The patriarchal system works if the patriarch supports the people in the system. But these days, if you admit that there's a family history of alcoholism, then suddenly you're not a candidate for opiates.
Or this doctor friend of mine who was recently suspended, he's a residency mate of mine. He had a mother and a daughter, both in chronic pain. I think they lived together and one was on hydrocodone and the other on oxycodone. And it turns out their medications may have gotten mixed up. They had a urinalysis and it looked bad.
But if you live in the same household, then maybe you can get your pills mixed up. I mean, you could have that chance. But do you fire somebody for having hydrocodone in their system? In my training, a lab result is something that you respond to, not something you react to. The first thing you do is you repeat the lab test. If you see something that's completely out of the ordinary, then you have to inquire about it.
But in this system, you have to fire the patient, even when there's no evidence that doing urine drug screens make any difference at all about diversion or patient care or anything. Nobody's done a placebo, double-blind controlled study about that.
I'm now seeing some pain refugees from Billings. The pain doctor there retired and the new guy came in slashing everybody’s dose and then he left. All these patients are trying to recover and none of them are oriented towards taking cannabis because they know that if they found cannabis in their urine that they would be fired. Somebody somewhere will say if you have cannabis in your urine, it's obvious that you're selling your pain pills in order to buy your cannabis. That is not obvious to me. Not even remotely
There’s a huge amount of gaslighting going on between the system and patients, that doctors are willing participants in. I mean, my younger colleagues have loans to pay off, so they are not risk takers. Right now, I am quite a risk taker. Because everything's gone. I don't have anything left.
I would say that in medicine today, the wheels are coming off and maybe they need to all come off. It doesn't operate in the same mentor-apprenticeship relationship that I was trained in. I don't know if I would last in medical training today.
I think that the internet has both good and bad things about it. And the good thing is that patients research their ailments or their symptoms and they come in with that. They come in with a differential diagnosis already. And I get to hear that and respond to it.
The great thing about that is what I call the alignment effect. If a patient comes to me and wants to do something that they read about and it's not going to kill them, if I say yes and it works for them, then they're going to be really persistent about following that therapy because it was their idea. Right? If it fails and they come back to me, then they're willing to listen to my point of view on it. And that builds trust.
If only the hospital administrator, pharmacist, insurance bureaucrat and legislator would trust our medical expertise. I think we're in a period of time where expertise is not thriving. Failing to use the expertise of our specialists is really putting us in a bad way, not only in medicine, but in the entire culture.
PNN: Thank you, Dr. Ibsen.