Justin Brown: A CRPS Story of Hope
/By Miles Ryan Fisher
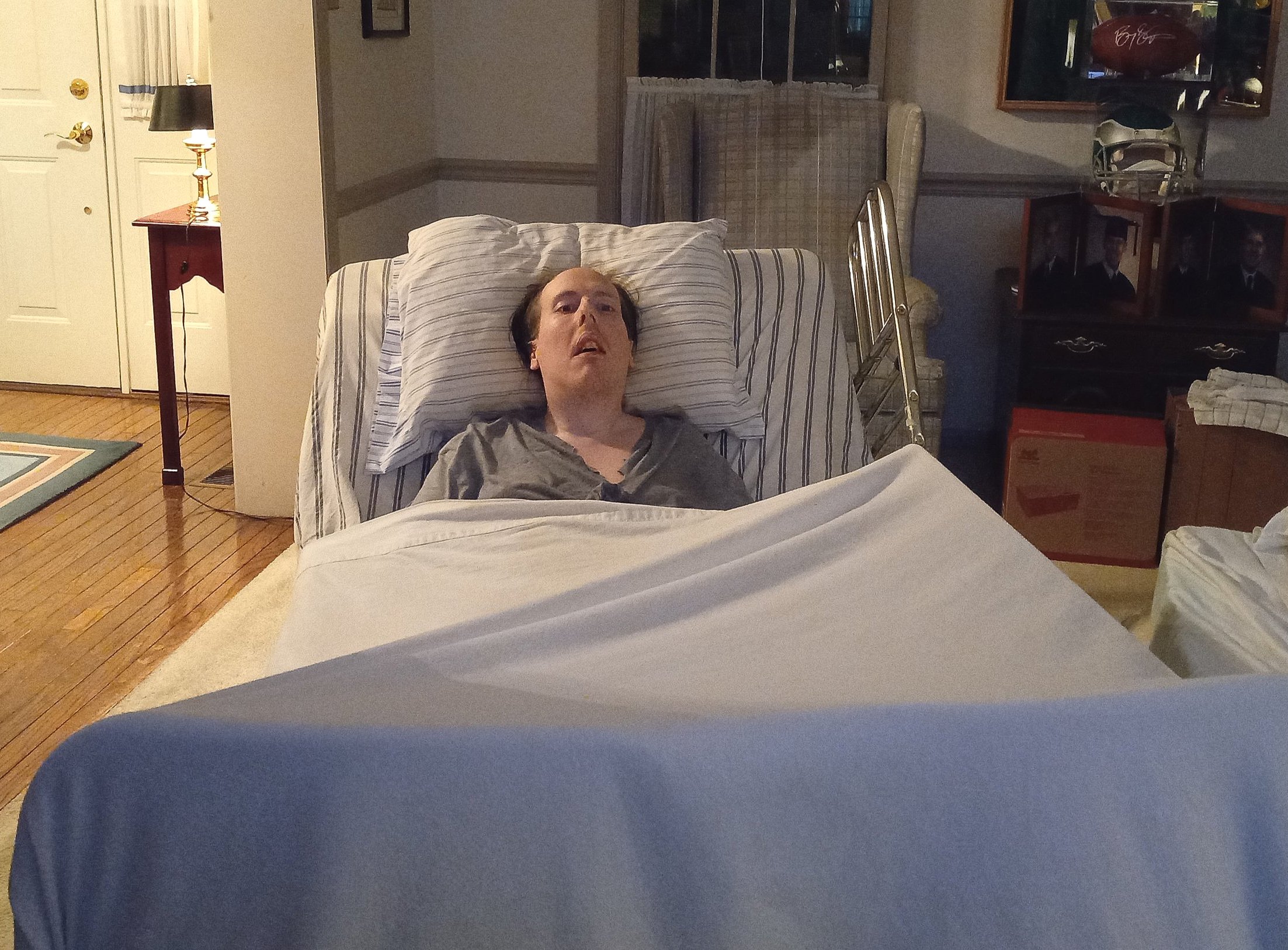
When Justin Brown took his first steps at the age of 40, his parents were overcome with joy. Only, it wasn’t the same joy that they’d experienced when he’d taken his first baby steps. No, this joy came with great pain — the kind of pain that comes with watching one’s child lose nearly half his life to a debilitating condition called Complex Regional Pain Syndrome, also known as “the suicide disease.”
In 2006, just as Justin prepared to enter the working world as a Penn State graduate, he started losing weight. He began regurgitating his meals, vomiting most of whatever he ate. Over time, he grew gaunt. His face sunk and his cheekbones protruded. His skin wrapped around his body until he looked emaciated.
Doctors didn’t have answers. When Justin reached the point that he couldn’t hold down any food, they inserted a J-tube — a feeding tube — in him so that food could bypass his stomach. But it wasn’t his stomach that was the problem. It was the parasite they hadn’t tested for — the one dwelling in his intestine right where the tube was inserted. When they removed the J-tube, they accidentally left a part of it in him.
When Justin awoke from surgery, he awoke to something even more unbearable. Something hellish. The operation triggered a pain that spread through his entire body and left him incapacitated, even after the parasite and tube remnant were removed. At the age of 26, Justin no longer had the strength to walk, not even to the bathroom.
Since 2007, he lay in a hospital bed in the middle of his parents’ living room in Fort Washington, Pennsylvania, his arms at his sides, his head always facing the same direction. In order to subdue the pain that incapacitates him, Justin takes a daily mixture of heavy pain medication, including narcotics.
It took many years until Justin and his family found a doctor who offered an accurate diagnosis: Complex Regional Pain Syndrome or CRPS. Only, the doctor didn’t call it that. Back then, the condition was known as Reflex Sympathetic Dystrophy or RSD.
CRPS/RSD happens when an injury — as minor as a broken finger or as major as surgery — triggers a pain so severe that it is, according to the McGill Pain Index, worse than than amputation. The pain typically remains in the region of the injury, usually involving a limb. But in Justin’s case, it spread through his entire body.
“A lot of people feel like their skin or their nerves are burning, but for me it feels like my bones are being crushed,” Justin says. “If I took my worst pain before CRPS, that would be like a 1 out of 10 compared to my pain now. You really can’t describe it.”
The pain that he’s bravely battled for 17 years has been excruciating and constant. With no end. And no cure.
“It’s there 24/7, and you don’t know when it’s going to go away or if it’s going to go away,” Justin says. “But I had two choices. One was to completely quit. And the other was to keep going and hope that it’ll get better.”
Finding Hope
But now, Justin is finally getting part of his life back through a form of non-allopathic (without drugs or surgery) treatment offered at the Spero Clinic in Fayetteville, Arkansas.
The clinic, which has over 40 employees and treats hundreds of patients every year, was founded in 2012 by Dr. Katinka van der Merwe. Born in South Africa, van der Merwe immigrated to the United States in 1994 and earned her Doctor of Chiropractic degree with the intent of using it to treat individuals who suffer from CRPS and other chronic pain conditions. .
Her clinic’s approach involves treating the vagus nerve, which is the longest and most complex of the body’s 12 cranial nerves. Individuals who suffer from chronic neurologic disorders often have an underactive vagus nerve, which causes inflammation that is either localized or, as in Justin’s case, envelops the entire body. It’s this inflammation that can cause excruciating pain.
“My philosophy and belief is that the body is incredibly intelligent and can heal from the inside out,” van der Merwe says. “People don’t come here to get a diagnosis and medication — they come here to have their bodies rehabilitated.”
The clinic approaches pain treatment in a holistic and noninvasive way, using a variety of therapies and tools involving electrical, physical and auditory/visual/sensory stimulation. It’s the clinic’s range of therapies that helps correct the nervous system and – hopefully -- puts the patient’s pain in remission.
“It’s a completely different approach to everything that I’ve tried so far,” Justin says. This has included the most radical of forms, such as being placed in a ketamine-induced coma in Mexico and brought out of it with the hope that his nerves would essentially reset. Some CRPS patients have found relief with ketamine infusions, but it didn’t work for Justin.
It was last March that Justin began treatment at the Spero Clinic. As soon as the first week ended, Justin experienced progress. It began with his ability to move his hands. Then the next week, he stood up. On the third week, he walked for the first time in 15 years.
Every week after that has brought similar victories — small to a healthy person, but momentous for Justin. Regaining his ability to drink a Gatorade. Regaining his ability to curl two-pound weights. Regaining his ability to wear clothing that fits him, rather than clothing so loose as to not press against his body and cause him a great deal of pain.
“Before I got here, the most I could take were fifteen steps,” Justin says. “And they weren’t good steps. I’d just drag my feet on the ground. Now I walk from my hotel room down a couple hallways, through the center of the hotel, and outside.”
Every incremental gain helps Justin continue to grind. Unlike most patients, who require two or three months of treatment, Justin will need at least half a year because of how severe his CRPS is.
Fundraising Help
The cost of every week of treatment — about $3,000 — is typically not covered by insurance, which does not make it any easier on Justin or his family. If anyone knows this, it’s Philip Robert, one of the Spero Clinic’s CRPS patients in 2016.
Robert spent ten weeks at the clinic and found his recovery so miraculous that he was inspired to form the Burning Limb Foundation, a non-profit whose mission is to raise funds to provide financial assistance to people with CRPS, primarily for treatment at the Spero Clinic. What makes the foundation different from most other non-profits is that 100% of the donations it receives are applied to treatment costs. And unlike other fundraising platforms like GoFundMe, donors are then able to write it off as a charitable gift on their tax returns.
“The idea is to get (CRPS patients) started — get them seed money — so that they can then do a fundraising campaign in the nonprofit world,” Robert says. “We provide a platform in which families can utilize their resources—their network of friends and family—who may be willing to give a little bit more.”
It’s through the Burning Limb Foundation that Justin has received much-needed financial support from family, friends and even people who have never met him but want to play a role in his recovery.
It’s that recovery that Justin realizes is so important, not only to live a life free of pain, but also to inspire others like him who suffer from CRPS. While not cured of the disease, he hopes his remission can bring hope to others.
“If it can work for me, it can work for anybody,” Justin says. “It’s not guaranteed to work for everybody, but it can work for anybody.”
Miles Ryan Fisher is the Assistant Director of the Building Trades National Medical Screening Program and also serves on the advisory board for Columbia Lighthouse for the Blind. His articles have appeared in the Washington Post, Philadelphia Inquirer, Washingtonian Magazine, Motherly, and Go World Travel.