FDA Approves Device That Uses Artificial Intelligence to Treat Chronic Pain
/By Pat Anson, PNN Editor
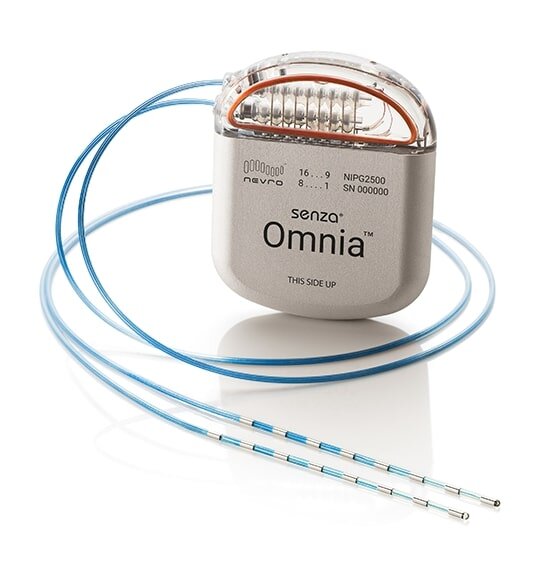
The Nevro Corporation says it has won approval from the Food and Drug Administration for an advanced spinal cord stimulator (SCS) that uses artificial intelligence to individualize treatment for each patient.
Nevro says its Senza HFX iQ stimulator “learns from patients” as they use the device and develops customized algorithms for treating chronic back pain, leg pain and pain from diabetic neuropathy.
"HFX iQ is designed to improve the consistency of pain relief and is the only SCS system that truly personalizes care," D. Keith Grossman, Chair and CEO of Nevro, said in news release.
"Pain is variable from patient to patient and over time. Using the big data from our HFX Cloud patient database, our unique HFX Algorithm was developed to identify those programs where patients have been more likely to get relief in the real world. HFX iQ takes direct input from each patient on their pain and quality of life measures to get smarter over time and recommend program changes.”
Nevro says patients will start with a program most likely to provide pain relief, and then adjusts it over time based on patient input and medical data, such as pain scores, activity levels and changes in use of pain medication. Patients can also adjust their pain relief programs through an app on their smartphones.
Spinal cord stimulators are usually considered the treatment of last resort for people with intractable or severe chronic pain that doesn’t respond to other therapies. The surgically implanted devices emit low levels of electricity that reduce the intensity of pain signals.
Unlike older stimulators, Senza stimulators use electric pulses of 10 kHz, a high frequency that doesn’t create an uncomfortable tingling sensation and delivers more pain relief. Last year the FDA approved Senza stimulators for the treatment of painful diabetic neuropathy, making it the first spinal cord stimulation system approved for that condition. Until then, most SCS devices were only approved for patients with severe back pain.
About 50,000 stimulators are implanted in the U.S. every year, with failure rates for the devices estimated at 25 to 30 percent. Most patients are required to undergo psychological testing and a trial treatment before getting a SCS.
The FDA has come under scrutiny for its regulation of stimulators and other medical devices. A 2020 report by Public Citizen accused the FDA of “dangerously lax” oversight of stimulators, which were linked to 156,000 injuries and 931 deaths. The agency responded to the report by sending a letter to healthcare providers reminding them to only implant stimulators after a trial period that demonstrates the device are effective. An FDA review of adverse events involving stimulators found that nearly a third were reports of unsatisfactory pain relief.
Nevro says it will begin a limited release of Senza HFX iQ later this year, with a full market launch in the U.S. in early 2023. Nevro is also seeking approval of HFX iQ in Europe.