By Alon Ironi, CEO, Theranica Bio-Electronics
Well into my adulthood, I struggled with chronic back pain. I took medication after medication, finding myself getting sucked into the habit of popping painkillers and wondering why my pain wasn’t healing. I eventually discovered the psychological and mental elements inherent in chronic pain, and shifted my approach to pain management, which rapidly cured my back pain.
The holistic approach to healing pain has historically been ridiculed in the medical community, preventing many physicians from recognizing the legitimacy of alternative treatments. The time has come to evolve beyond just popping pills to treat pain, towards a biopsychosocial perspective.
The discovery and introduction of penicillin in 1928, marked the very beginning of medication popularization in the West. The development of new medications – for a wide range of uses - has extended life span and improved quality of life. Unfortunately, the benefits associated with medication have encouraged its frequent use for disorders it simply is not meant for, such as certain types of chronic pain.
Medication overuse headache is one example where drugs intended to treat migraine and headache can, with excessive use, lead to the deterioration of the exact condition they are supposed to be treating. Medication is an incredible tool when used properly, but it’s not the only tool, and it can be seriously harmful when misapplied.
What is Pain and How Do We Treat It?
Pain is a unique bodily experience that, unlike other disorders, indicates an underlying issue in one’s physiology. Pain is an alarm system. It tells us that something is wrong, and if we mask it without treating its underlying cause, we might cause a great disservice to our bodies.
The use of medication to treat short-term acute pain, while a person simultaneously heals from the cause of that pain, like a pulled muscle or a tear in a tissue, makes sense. However, the use of medication in instances of chronic pain - pain that persists longer than three months - is problematic.
Chronic pain is a debilitating condition that impacts an individual’s everyday life. From migraine to chronic knee pain to chronic back pain, the routine of normal life is disrupted. Often, this chronic pain had an initial cause, such as a surgery, fall or injury that has since healed, but the pain persists long after its source has disappeared. This type of pain, as Haider Warraich, a physician and clinical researcher at Harvard, so aptly puts it, is like “an overlearned traumatic memory that keeps ricocheting around in our brains, often long after the injury it rehearses has fully healed.”
This perception of chronic pain has its roots in quite a controversial physician -the late Jon Sarno, MD, a professor of rehabilitation medicine in the 1980’s and 90’s. His theories, while not rigorously proven in formal clinical studies, were built upon anecdotal data from thousands of patients he treated during his lifetime and are still being explored today. They have jumpstarted a revolution in our understanding of pain.
The biopsychosocial model focuses on illness as a complex interaction of chemical and electrical reactions that are induced by biological, psychological and social factors. Contemporary pain researchers, like Lorimer Mosely, a clinical scientist, have applied this model to pain, recognizing that pain is comprised of both physical sensation and emotional stimulus, such as the fear of pain itself.
Pain is no longer perceived as entirely “physical” in nature. It is now understood to be exacerbated by the fear of tissue damage and the aversion to previously experienced pain. As clinical research develops and shifts its focus to a more biopsychosocial approach to illness and pain, doctors must re-evaluate their first-line treatment suggestions.
Drug-Free Pain Management
Based on this new perception of pain, several nonpharmacological treatment methods for pain management have been developed. One approach is a purely psychological treatment called cognitive behavioral therapy, a form of talk therapy that discourages negative thoughts associated with pain and trains people to adhere to thoughts that stimulate the body’s natural pain relief system. Another example of nonpharmacological pain treatment is massage therapy, which addresses pain by releasing muscle tension.
Neuromodulation is highly effective in treating certain pain disorders through the use of electrical stimulation to modulate pain pathways in the neural system. Several forms of neuromodulation treatment exist today, with different mechanisms of action and efficacy.
Spinal cord stimulation, for example, is used to treat back pain and leg pain. But it is highly invasive, with electrodes surgically placed near the spinal cord to send electrical currents to the spine.
Deep brain stimulation is being studied for the relief of chronic pain, but it is also quite invasive, as it involves implanting electrodes into the brain.
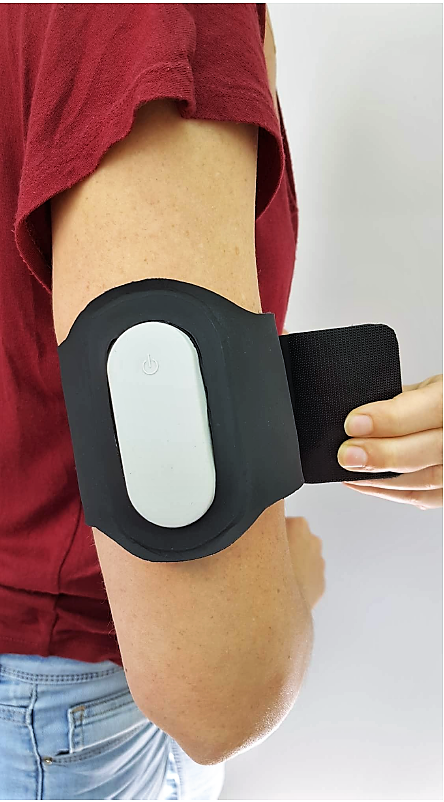
Nerivio is a non-invasive, wearable neuromodulation device made by my company that is FDA-approved for the treatment of acute migraine. Nerivio is self-applied to the upper arm, where it uses remote electrical neuromodulation (REN) to stimulate analgesic neurotransmitters in the pain pathways of migraine. In clinical trials, Nerivio and other REN devices have been shown to be just as effective as pharmacological treatments.
To be clear, medication is a necessary and beneficial tool for treating infections, reducing fever, managing sickness and much more. However, its use in chronic pain management is sometimes misplaced, especially at a time when newer non-drug therapies are emerging.
The holistic approach to pain management is the future. It considers the balance and context of a patient’s life and combines multiple modalities for their treatment. People are multifaceted and their treatment should be multifaceted as well. It is my hope and vision that this field of research will continue to develop and will soon be widely embraced by most medical professionals.
Having experienced the benefits of drug-free pain management first-hand, I truly hope that health care systems will support patients in accessing these much-needed alternative treatments to improve quality of care and life.