FDA Approves First Marijuana-Based Prescription Drug
/By Pat Anson, Editor
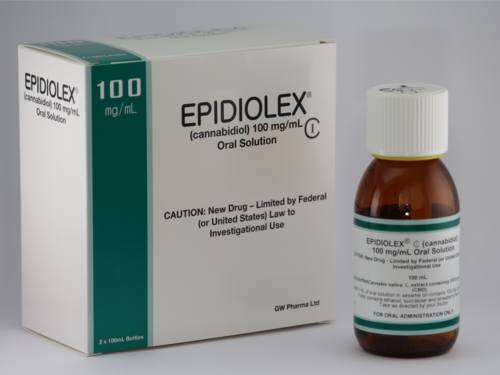
The U.S. Food and Drug Administration has approved the use of Epidiolex, the first drug derived directly from marijuana, to treat seizures caused by two rare and severe forms of childhood epilepsy, Lennox-Gastaut syndrome and Dravet syndrome.
Epidiolex is the first FDA-approved medication that contains cannabidiol (CBD), one of the active ingredients in marijuana. It does not contain tetrahydrocannabinol (THC), the chemical compound in marijuana that makes people high.
“This is an important medical advance. But it’s also important to note that this is not an approval of marijuana or all of its components. This is the approval of one specific CBD medication for a specific use. And it was based on well-controlled clinical trials evaluating the use of this compound in the treatment of a specific condition,” FDA commissioner Scott Gottlieb, MD, said in a statement.
FDA approval of Epidiolex is a major milestone for GW Pharmaceuticals, a British company focused on developing CBD-based medications. The company said Epidiolex would be available in the fall, but did not disclose the price. Some analysts have predicted it could cost as much as $25,000 a year.
Many oils and tinctures containing CBD are already sold online and in states were medical marijuana is legal, but the FDA has not approved any of them. The agency has only approved a handful of synthetic cannabinoids such as Marinol (dronabinol) to treat loss of appetite and nausea.
“We’ll continue to support rigorous scientific research on the potential medical uses of marijuana-derived products and work with product developers who are interested in bringing patients safe and effective, high quality products,” Gottlieb said.
“But, at the same time, we are prepared to take action when we see the illegal marketing of CBD-containing products with serious, unproven medical claims. Marketing unapproved products, with uncertain dosages and formulations can keep patients from accessing appropriate, recognized therapies to treat serious and even fatal diseases.”
Some children in clinical trials experienced side effects from Epidiolex such as liver toxicity, anemia and drowsiness, but an FDA staff report said the risks were “mild to moderate” and could be managed with warning labels. The staff report also found there was low risk of the strawberry flavored Epidiolex being abused.
“Today’s approval of Epidiolex is a historic milestone, offering patients and their families the first and only FDA-approved CBD medicine to treat two severe, childhood-onset epilepsies,” Justin Gover, GW Pharmaceutical’s CEO, said in a statement.
“This approval is the culmination of GW’s many years of partnership with patients, their families, and physicians in the epilepsy community to develop a much needed, novel medicine. These patients deserve and will soon have access to a cannabinoid medicine that has been thoroughly studied in clinical trials, manufactured to assure quality and consistency, and available by prescription under a physician’s care.”
While Epidiolex is only approved for the treatment of Lennox-Gastaut syndrome and Dravet syndrome, doctors will presumably be able to prescribe it “off label” for other conditions such as chronic pain.
GW Pharmaceuticals also makes Sativex, an oral spray that contains both CBD and THC. Sativex has been approved in Europe, Canada, Australia, New Zealand and several other countries for the treatment of muscle spasticity caused by multiple sclerosis. In Israel, Sativex is also approved for the treatment of pain and chronic non-cancer pain.